What are you looking for?
Search
Devolux® PLLA Filler Instruction Manual
Please be sure to read the instructions carefully before use and follow the directions.
Devolux® PLLA Filler Composition
Each vial contains:
Poly-L-lactic acid 160mg
Mannitol 152mg
Sodium carboxymethyl cellulose 48mg
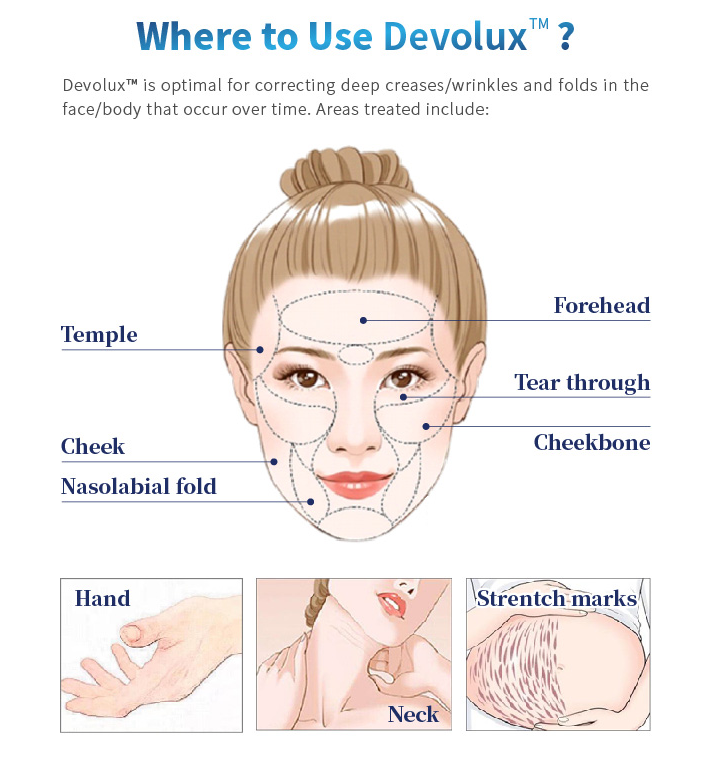
Targets
Face(Forehead, Temple, Tear through, Cheekbone, Cheek, Nasolabial fold) , Hand, Neck, Strentch marks
Mode of action
Devolux® is a synthetic, biodegradable injectable plla filler, which is composed of microparticlebeads of poly-L-lactic acid (PLLA). PLLA is a biocompatible, biodegradable, synthetic polymer.which is a derivative of the alpha hydroxy acid family. Devolux® treats the visible signs of aging onthe face, by restoring lost facial volume, and treating shallow and deep facial lines. The mecha-nism of action of Devolux® works by stimulating the body to incur collagen production. Devolux® injections create new collagen and elastin in the skin, firm and tighten the skin, and restore lostvolume.
Process of taking effect
After the injection is completed, Devolux® immediately fills the depressed defects, and at this time, the wrinkles and depressions are filled. Within 3 to 4 days after the initial treatment, the water content in the used material is absorbed by the body, and the depressions may slightly collapse again, but please do not panic, this is a normal phenomenon. After 7 to 14 days post-injection, Devolux® stimulates the body to produce collagen, and the wrinkles and depressions begin to fill. Approximately four weeks later, the improvement becomes progressively noticeable, but deeper depressions and wrinkles may require further treatment.
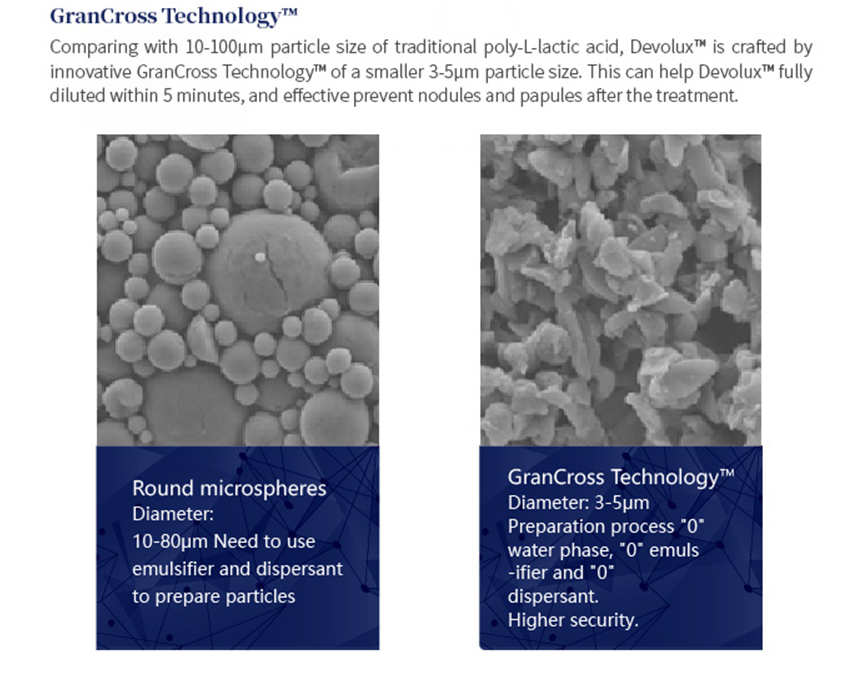
GranCross Technology™
Comparing with 10-100um particle size of traditional poly-L-lactic acid, Devolux® is crafted byinnovative GranCross Technology™ of a smaller 3-5um particle size. This can help Devolux® fullydiluted within 5 minutes, and effective prevent nodules and papules after the treatment.
Reconstitution method:
1. Dissolve 160mg in 8ml of sterile water for injection.
2. Allow to dissolve for 5 minutes.
3. Add 2ml of lidocaine.
4. After shaking well, draw up for use. Recommended needles: 27G and 30G sharp needles, or 27G blunt needle.
Treatment level
Between the subdermis and the superficial fat pad.
Treatment course
Three treatments per course, with follow-up visits in the 1st, 2nd, and 4th month, supplemented as needed, small amounts multiple times, with one course lasting 2-3 years.
Method of use
Forehead
• Dosage: 5-8ml
• Application Level: Blunt needle spread primarily subcutaneously, with periosteum as secondary.
• Note: If the dosage is too large, the water content will flow downwards.
Temples
• Dosage: 2-3ml per side
• Application Level: Use blunt and sharp needles in combination, mainly in the subcutaneous fat layer and temporal muscle layer.
• Note: After withdrawing the needle, press until the medication is evenly dispersed. Then use a blunt needle to evenly spread in the subcutaneous fat layer and press evenly.
Cheekbones
• Dosage: 2ml per side
• Application Level: It is recommended to use a blunt needle spread in multiple layers including subcutaneous, fat layer, fascia layer, subfascial tissue, and above the periosteum.
Tear Troughs
• Dosage: 1ml per side
• Application Level: Sharp needle is recommended, used at single points in the subcutaneous and fat layer.
Nasolabial Folds
• Dosage: 0.3—1ml per side
• Application Level: Use blunt and sharp needles in combination, with the blunt needle evenly spreading at various depths of the nasolabial fold. Use below the nasolabial fold to avoid dispersion.
• Note: Try not to make exaggerated expressions for three days after use to prevent muscle pressure from dispersing the medication upwards. Additionally, for the nasal base, it is recommended to use a sharp needle vertically.
Cheeks
• Dosage: 3-4ml per side
• Application Level: It is recommended to use a blunt needle spread in multiple layers including subcutaneous, fat layer, fascia layer, subfascial tissue, and above the periosteum.
Hands
• Dosage: 10-20ml per hand
• Application Level: Subcutaneous layer
Neck
• Dosage: 1.5-3ml per neck line
• Application Level: Between the subdermis and the platysma, both 30G sharp needle or 27G blunt needle can be used.
Stretch Marks
• Dosage: 20ml-30ml
• Application Level: At the site of obvious layer rupture, sharp needle vertically into the deep dermis.
• Note: Do not inject into the fat layer.
Postoperative nursing
• Apply ice to the treated area several times over 24 hours to help reduce swelling.
• Self-massage the treated area starting on the 4th day after the procedure, continue for 3 days, massaging 3 times a day, for 3 minutes each time.
• Avoid exposure to sunlight and UV lamps.
Contraindications
• Relative contraindications: autoimmune diseases, systemic lupus erythematosus, vitiligo.
• Strict contraindications: severe scar constitution.
• Individuals under the age of 18 should not use it.
• Patients with a history of allergies to any ingredient of the product should not use it.
• Patients allergic to lidocaine or other amide-type local anesthetics should not use it.
• Patients with a history of severe systemic allergic reactions, or those who have had or have multiple severe allergies, should not use it.
• Do not use this product when the area to be treated, or a nearby area, has an active disease, such as inflammation (skin eruptions, such as boils, acne, rashes, or hives), infection, or tumors, until the condition is under control.
Warning
• Devolux® should only be used in the deep dermis, bone, or subcutaneous tissue.
• Be sure to stop the administration before the needle is with-drawn, and focus on massaging at the needle entry point tospread the medicine, so as to avoid excessive drug residue atthe needle entry point and increase the risk of nodules.
• This product must not be injected into muscles or veins. Injection into or near blood vessels can cause local superficial death and scarring, which is caused by damage, blockage, or injury to the vessels. Special care should be taken if the patient has had surgery in the area to be treated. Areas with limited blood flow from collateral vessels may increase the risk of ischemia. Aspiration is recommended before injection.
• Accidental placement of soft tissue fillers into facial blood vessels may lead to embolism, vascular occlusion, ischemia, necrosis, or infarction of the implanted site or the area supplied by the affected vessels. Rare but serious adverse events include temporary or permanent visual impairment, blindness, cerebral ischemia or cerebral hemorrhage leading to stroke, skin necrosis, and potential damage to underlying facial structures. If any of the following symptoms occur, stop the injection immediately, including changes in vision, stroke-like symptoms, skin whitening, or unusual pain during or shortly after treatment. The patient should receive immediate medical care and be evaluated by a physician to determine if the injection was into a vessel.
• Pay attention not to excessive treatment, just reach the idealstate, do not overdose, reduce the occurrence of swelling.
• Devolux® vials are for single-patient, single-use only to avoid contamination. Vials should not be reused or resterilized. Dispose of immediately after use. Do not use if the packaging or vial has been opened or damaged.
• The powder must be reconstituted with sterile water for injection.

F&Q
▪ How to inject to get a better effet?
The blunt needle is stripped, and the suspension isinjected widely with multiple layers and multipletunnels. The blunt needle peeling method is easierthan the sharp needle treatment, and the treatmenteffect will be better, the tissue swelling caused bythe peeling will generally be relieved after 72 hours,and the patients can also be recommended to takethe "Caomuxiliu Infusion Tablet" produced in Japan,also known as "Eliminate". After injection, massage(use the palm root position) to make the productrelatively fixed in the interstitial space. Aftermassage, 20-30 units of botulinum toxin injection can beused to assist positioning.
▪ Can I exercise after the procedure?
You can resume exercise the next day. The product is stable, and the human body has a short recovery period after treatment. Do not wet the treated area for 6-24 hours post-treatment. The risk of infection mainly comes from the skin channel (needle hole) during treatment.
▪ What causes the product to clump? How should it be handled?
Long-term exposure to high temperatures during transport or storage, or violent shaking, may cause the product to clump. You can add saline and let it sit for 10 minutes to see the dilution effect; if there is still a lot of sediment, you can use the supernatant for treatment. If the doctor determines it cannot be used, you can provide, but not limited to: clear pictures of the suspension from multiple angles, packaging + batch number, time and place of use, and a brief description of the usage process. After confirmation by the company's designated quality control personnel, a replacement can be arranged.
▪ Can it be combined with photonic projects/BO?
It can be combined. It is recommended to do the youth needle first, and then photonic projects 4-6 months later (photonic projects generally last 30-40 minutes, and the temperature is high, while PLLA decomposes faster at 50-60 degrees Celsius, so if the youth needle is done first, followed by photonic projects, the effect of the youth needle will be compromised. If you want to do both at the same time, you can stagger the areas, or do the photonic project first, and after cooling the local skin for half an hour, proceed with Devolux treatment.
It can also be combined with BO treatment, as BO can block the transmission of information between nerves and muscles, which is more conducive to the fixation of PLLA.
▪ Can it be used for acne scar treatment?
Yes, generally 0.2-0.3ml per 1 square centimeter can be used.
▪ What to do if nodules and swelling occur?
Nodules can dissolve over time. Try not to treat and allow it to recover on its own if a reaction occurs.
▪ What type of lidocaine is used to dissolve PLLA?
Ordinary lidocaine is sufficient; adrenaline lidocaine should not be used.
Expiry time and storage conditions
Expiry time: 24 months
Storage conditions: Devolux® should be stored at room temperature and away from heat sources (not exceeding 30°C). Once reconstituted, Devolux® can be stored in the refrigerator (2-8°C) or at room temperature not exceeding 30°C for up to 72 hours.
If the product has exceeded the manufacturing date and shelf life indicated on the packaging, or if the batch number is missing or difficult to identify, please do not use it.
Do not freeze.
After use, the treatment syringes and needles may pose a potential biological hazard. They should be disposed of in accordance with approved medical practices and applicable local regulations.
If you are interested in Devolux® plla filler, you are welcome to click here to contact us.








 https://www.facebook.com/profile.php?id=61560092512129
https://www.facebook.com/profile.php?id=61560092512129